Pharmaceutical & Healthcare
Safeguard Your
Temperature-Sensitive
Pharmaceutical Products




MONITORING
Guaranteeing the quality and integrity of your temperature-sensitive items
For pharmaceutical shipments, ensuring their quality requires monitoring of location and temperature during transit. Tempmate provides a solution that offers verified visibility into these critical shipments. This empowers you to curtail excursions and uphold product excellence from start to finish.
MONITORING
Tempmate offers diverse monitoring solutions
to ensure optimal transportation conditions
- Ensure Quality and Traceability by supervizing a wide range of temperatures: dry ice (-78.5°C), frozen (-25°C to -15°C), refrigerated (+2°C to +8°C) or controlled room temperature (+15°C to +25°C)
- Prevent Loss with alarms based on configurable thresholds
- Optimize on-time deliveries thanks to localisation

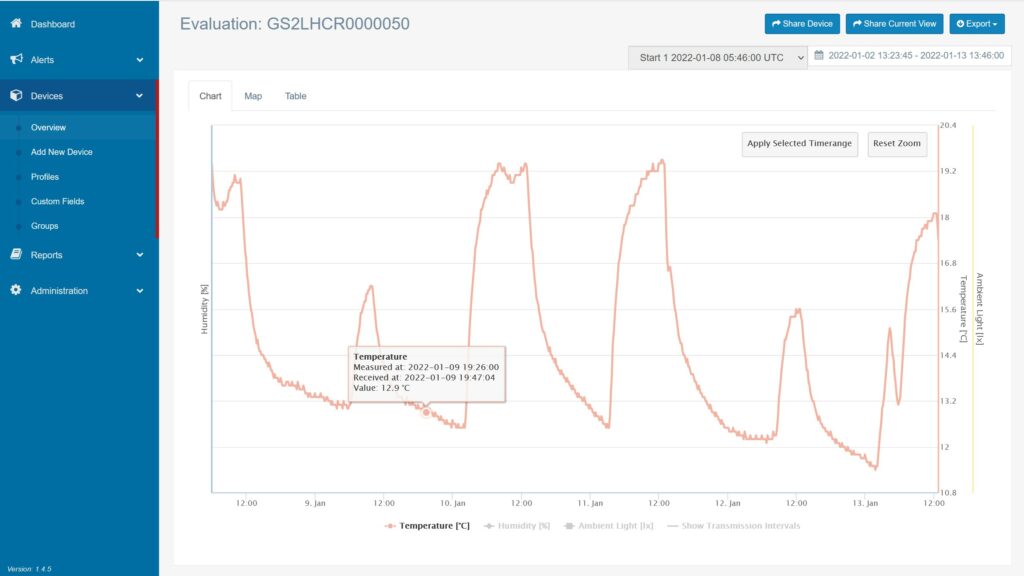
Demonstrating Regulatory Compliance
Staying in line with standards and regulations is crucial for both assuring product quality and avoiding legal repercussions.
- Good Distribution Practices (GDP) compliance
- Temperature recorders in accordance with EN12830 standard
- Every data logger includes an individual 6-point temperature validation certificate
- Software with audit trail and CFR21 Part 11 compatibility


PRODUCTS
A solution for every need


tempmate.®-S1 PRO:
USB temperature data logger
Ensure the integrity of your supply chain with the tempmate.®-S1 PRO, a reliable and user-friendly USB temperature data logger that safeguards your temperature-sensitive products.

tempmate.®-GS2:
4G Real-time temperature data logger


Daniel Fiedrich
Product Specialist
We’d love to hear from you.
You are currently viewing a placeholder content from reCAPTCHA. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information